As we all know, the cycle life of batteries is limited. When it is used for a period of time, it will appear that after charging, it cannot be used for a longer time than when it was originally bought. This is due to the attenuation of battery capacity. The battery capacity attenuation can be detected by the battery charge and discharge tester. Once the battery capacity attenuation is found, we can repair the battery in time. What is battery capacity fading? Why does battery capacity decline?

Mobile phone batteries have aging problems. It has a limit for the number of charging and discharging cycles, generally between 300 and 500 times. The charging and discharging cycle refers to charging the battery from 0% to 100%, and then discharging from 100% to 0%. the complete process.
When the number of cycles is getting closer and closer to the limit value, the internal resistance of the battery will increase, that is, the battery capacity will begin to decay, and the battery loss will become more serious, so that the battery capacity will become less and less durable.
Why does battery capacity fade?
As the manganese ions (gray) are pulled out of the battery's positive terminal (blue), they react with the electrolyte (gold) near the battery's negative terminal, trapping lithium ions (green/yellow).
When a battery enters the "old" stage, scientists will name the phenomenon of its weakened performance as "capacity fading". The phenomenon of "capacity fading" refers to the phenomenon that the battery capacity gradually decreases as the number of times the battery is charged increases. A mobile phone can last for a whole day when it is first used, but it can only last for a few hours after two years. The reason for this phenomenon is capacity decay.
So what if scientists could slow down the process of capacity fading and allow batteries to age more gracefully?
Researchers at the U.S. Department of Energy's Argonne National Laboratory said in an article published in the Journal of the Electrochemical Society that they have successfully confirmed a major cause of capacity fading in high-energy storage lithium-ion batteries.
For lithium batteries -- the kind we use in our laptops, smartphones, plug-in hybrid cars -- its capacity is closely tied to the number of lithium ions it can switch back and forth between its two poles as it charges and discharges.
The back-and-forth conversion of lithium ions is excited by specific transition metals, which change the oxidation state of lithium ions as they flow through the positive electrode of the primary battery. However, as the battery cycles, some of the ions -- especially manganese ions -- are stripped out of the battery's positive electrode and end up attached to the battery's negative electrode material.
Once close to the battery's negative electrode, these metal ions interact within the "solid electrolyte membrane" region of the battery. The solid electrolyte membrane is formed by the interaction of a highly active anode with a liquid electrolyte that carries lithium ions back and forth. For each electrolyte molecule that reacts to break down in a process known as a reduction reaction, lithium ions become trapped in the interphase. As more and more lithium ions are trapped in the interphase, the energy storage capacity of the battery gradually decays.
On the solid electrolyte membrane, some molecules are not fully reduced, which means they can accept more electrons and combine with more lithium ions. The particles are like flammables waiting for a pinch of fireworks.
When these manganese ions are attached to the surface of these particles, they are like fireworks sprinkled on combustibles: the lithium ions are a powerful catalyst for chemical reactions that incompletely degrade the molecule, in the process putting more Lithium ions are trapped in the interphase.

"There is a strong correlation between the amount of manganese ions attached to the negative electrode of the battery and the amount of trapped lithium ions," said Daniel Abraham, an Argonne scientist and study co-author. By understanding the mechanisms behind trapping and capacity fading, we were able to set out to find solutions to the problem."
 English
English
 Español
Español
 Português
Português
 русский
русский
 français
français
 日本語
日本語
 Deutsch
Deutsch
 Tiếng Việt
Tiếng Việt
 Italiano
Italiano
 Nederlands
Nederlands
 Pilipino
Pilipino
 Türk
Türk
 Gaeilge
Gaeilge
 عربى
عربى
 Indonesia
Indonesia
 norsk
norsk
 čeština
čeština
 Ελληνικά
Ελληνικά
 فارسی
فارسی
 தமிழ்
தமிழ்
 Српски
Српски
 Català
Català
 עִברִית
עִברִית
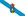 Galego
Galego
 Беларус
Беларус
 Hrvatski
Hrvatski
 ជនជាតិខ្មែរ
ជនជាតិខ្មែរ
 Кыргыз тили
Кыргыз тили
 O'zbek
O'zbek
 Lëtzebuergesch
Lëtzebuergesch
 ไทย
ไทย
 Polski
Polski
 한국어
한국어
 Svenska
Svenska
 magyar
magyar
 Malay
Malay
 বাংলা
বাংলা
 Dansk
Dansk
 Suomi
Suomi
 हिन्दी
हिन्दी
 български
български
 ລາວ
ລາວ
 Latine
Latine
 Қазақ
Қазақ
 Euskal
Euskal
 Македонски
Македонски
 Lietuvos
Lietuvos
 Eesti Keel
Eesti Keel
 Română
Română
 मराठी
मराठी




